2021 DSE 化学-Chemistry 真题 答案 详解
2021-05-03 dse dse 化学-Chemistry
| 序号 | 文件列表 | 说明 | ||
|---|---|---|---|---|
| 1 | 2021-化学-Chemistry-answer-zh.pdf | 10 页 | 2.70MB | 答案(中文) |
| 2 | 2021-化学-Chemistry-answer-eng.pdf | 16 页 | 8.94MB | 答案(英文) |
| 3 | 2021-化学-Chemistry-paper1-zh.pdf | 28 页 | 6.41MB | 真题 Paper 1(中文) |
| 4 | 2021-化学-Chemistry-paper2-zh.pdf | 6 页 | 1.70MB | 真题 Paper 2(中文) |
| 5 | 2021-化学-Chemistry-paper1-eng.pdf | 32 页 | 6.16MB | 真题 Paper 1(英文) |
| 6 | 2021-化学-Chemistry-paper2-eng.pdf | 8 页 | 1.02MB | 真题 Paper 2(英文) |
答案(中文)
解卷參考
本文件供閱卷員參考而設,並不應被視為標凖答案。考生及沒有參與評卷工作的教師在詮釋文件內容時應小心謹慎。
化學科
卷一
甲部
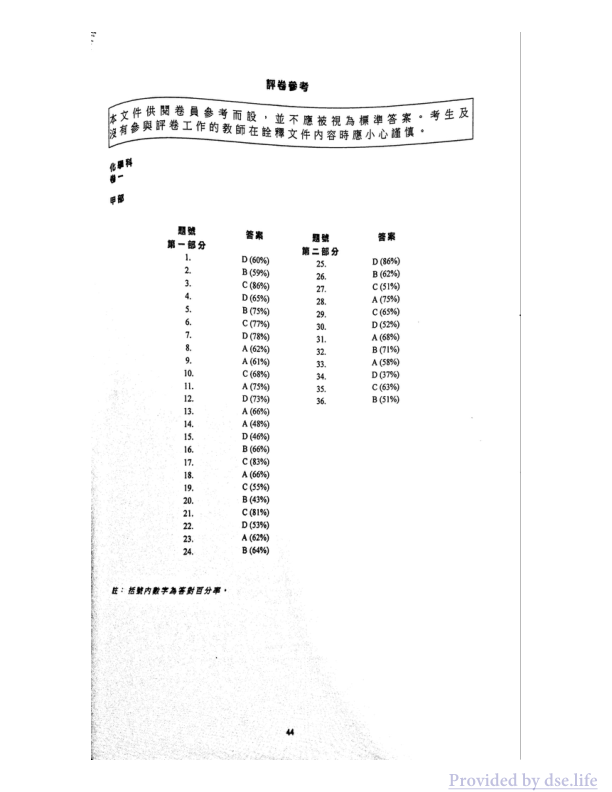
題號 答案 题号 答案
第一部分 第二部分
1. D(60%) 25. D(86%)
2. B(59%) 26. B(62%)
3. C(86%) 27. C(51%)
4. D(65%) 28. A(75%)
5. B(75%) 29. C(65%)
6. C(77%) 30. D(52%)
7. D(78%) 31. A(68%)
8. A(62%) 32. B(71%)
9. A(61%) 33. A(58%)
10. C(68%) 34. D(37%)
11. A(75%) 35. C(63%)
12. D(73%) 36. B(51%)
- A(66%)
- A(48%)
- D(46%)
- B(66%)
- C(83%)
- A(66%)
- C(55%)
- B(43%)
- C(81%)
- D(53%)
- A(62%)
- B(64%)
注:括號内數字為答對百分率
44
Provided by dse.life
答案(英文)
機密(只限閱卷員使用)
CONFIDENTIAL (FOR MARKER'S USE ONLY)
Part I
- (a) H C H | | | H C H Marks 1 (Accept answer with correct inner shell electrons) (Not accept answer with incorrect inner shell electrons, if inner shell electrons are drawn)
(b) 2C2H2(g) + 502(g) → 4CO2(g) + 2H2O(l) (State symbols not required)(Ignore incorrect state symbols) 1
(c) (i) hydrogen / H2 1 (ii) Hydrogen is explosive / flammable. 1
(d) Ca(OH)2 can be used in treating acidic soil / use in scrubber / treating acidic flue gas / treating acidic sewage / treating sewage by precipitation / making preserved eggs / making cement or concrete / etc. 1
- (a) (Reddish) brown (fume / liquid) (Accept: orange; Not accept: yellow or red; Not accept: solid) 1
(b) Pb2+(l) + 2e- → Pb(s) (State symbols not required)(Ignore incorrect state symbols) 1
(c) 4OH-(aq) → 2H2O(l) + O2(g) + 4e- (State symbols not required)(Ignore incorrect state symbols) (For (b) and (c), not accept “→”, “—”, and “e”) (For (b) and (c), deduct 1 mark if any one of “→”, “—”, and “e” is used in both parts, and all others are correct)
(d) hydrogen / H2 1
(e) Cu2+(aq) / copper(II) ion 1
(f) Brown solid formed. / The electrode increased in size. / The electrode increased in mass. 1
(g) Cu2+ is lower than H+ in electrochemical series. / Cu2+ is a stronger oxidising agent than H+. / Cu2+ accepts electrons more readily than H+. (Accept: state electrochemical series as E.C.S.) (Not accept: symbols without charges, like: Cu is lower than H in electrochemical series) (Not accept: Cu2+ is a better oxidising agent than H+) 1
2021-DSE-CHEM 1 & CS(CHEM) B-3
Provided by dse.life

真题 Paper 1(中文)
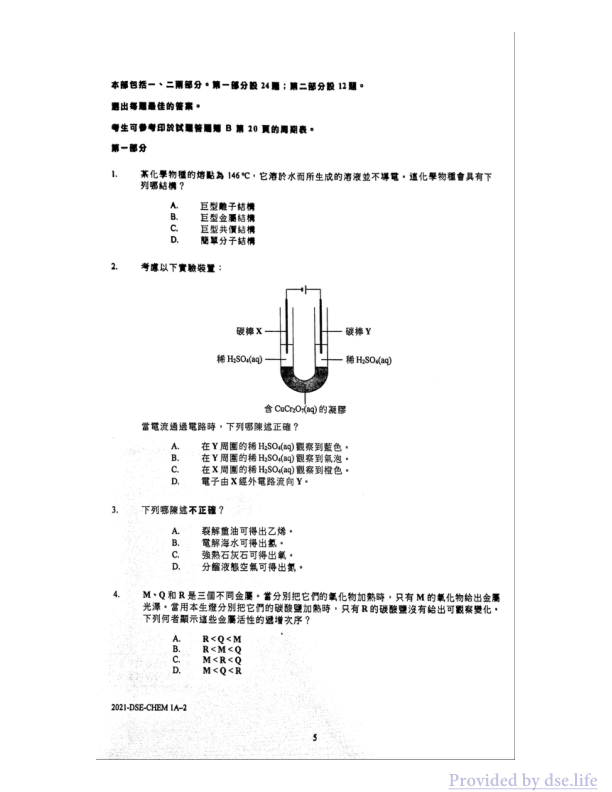
本部包括一、二两部分·第一部分設24题;第二部分設12題。
提出每题最佳的答案。
考生可参考印試圖答题頁B第20頁的周期表。
第一部分
1.
某化學物種的熔點為146°C,它溶於水而所生成的溶液並不導電·這化學物種會具有下列哪結構?
A. 巨型離子結構
B. 巨型金屬結構
C. 巨型共價結構
D. 隔單分子結構
2.
考慮以下實驗裝置:
當電流通過電路時,下列哪陳述正確?
A. 在Y周圍的稀H₂SO₄(aq)觀察到藍色。
B. 在Y周圍的稀H₂SO₄(aq)觀察到氣泡。
C. 在X周圍的稀H₂SO₄(aq)觀察到橙色。
D. 電子由X經外電路流向Y。
3.
下列哪陳述不正確?
A. 裂解重油可得出乙烯。
B. 電解海水可得出氫。
C. 強熱石灰石可得出氧。
D. 分餾液態空氣可得出氮。
4.
M、Q和R是三個不同金屬。當分別把它們的氧化物加熱時,只有M的氧化物給出金属光泽。當用本生燈分别把它的碳酸鹽加熱時,只有R的碳酸鹽沒有給出可觀察變化。下列何者顯示這些金屬活性的遞增次序?
A. R < Q < M
B. R < M < Q
C. M < R < Q
D. M < Q < R
2021-DSE-CHEM 1A-2
5
Provided by dse.life
真题 Paper 2(中文)
甲部 工業化學
回答試題的所有部分。
- (a) (i) 在某些條件下,HCOOH(l) 如下所示分解成 CO(g) 和 H2O(I) 的活化能是 +77.7 kJ mol^-1. HCOOH(l) ⇌ CO(g) + H2O(I) ΔH = +28.5 kJ mol^-1 在相同條件下,由 CO(g) 和 H2O(I) 生成 HCOOH(l) 的活化能是多少(以 kJ mol^-1 為單位)? (1 分)
(ii) 某反應的活化能是 +65.0 kJ mol^-1,該反應在 27°C 時的速率常數為 ki 。計算這反應在 37°C 時的速率常數(以 ki 表示)。 (氣體常數 R = 8.31 J K^-1 mol^-1; 阿列爾斯方程:log k = 常數 - Ea / 2.3RT) (2 分)
(iii) 以下給出在某些條件下,反應 A(g) + B(g) → C(g) 的速率方程,其中 k2 是速率常數: 速率 = k2 [A(g)] [B(g)]^2/3 (1) 對於 B(g) 的反應級數是多少? (2) 這速率的單位是 mol dm^-3 s^-1 。寫出 k2 的單位。 (2 分)
(b) 下圖顯示怎樣利用哈柏法生產液態氨。 N2(g) — 净化器 — 反應室 — 冷凝器 — NH3(l) H2(g) — 净化器 — 未起反應的 N2(g) 和 H2(g)
(i) 解釋為什麼 N2(g) 和 H2(g) 在進入反應室前需要淨化。 (1 分)
(ii) 解釋為什麼把未起反應的 N2(g) 和 H2(g) 再次通回反應室。 (1 分)
(iii) 為什麼在冷凝器內的氨變為液體,但其氫離子則否? (1 分)
(iv) 在哈柏法的反應中使用催化劑。 (1) 提出一個可使用的催化劑。 (2) 試以麥克斯韋-波茲曼分布曲線,解釋為什麼當使用了催化劑後反應速度加快。 (5 分)
1-DSE-CHEM 2-2
Provided by dse.life

真题 Paper 1(英文)
2021-DSE CHEM PAPER 1A
HONG KONG EXAMINATIONS AND ASSESSMENT AUTHORITY HONG KONG DIPLOMA OF SECONDARY EDUCATION EXAMINATION 2021
CHEMISTRY PAPER 1
8:30 am - 11:00 am (2 hours 30 minutes)
This paper must be answered in English
GENERAL INSTRUCTIONS
-
There are TWO sections, A and B, in this Paper. You are advised to finish Section A in about 45 minutes.
-
Section A consists of multiple-choice questions in this question paper, while Section B contains conventional questions printed separately in Question-Answer Book B.
-
Answers to Section A should be marked on the Multiple-choice Answer Sheet while answers to Section B should be written in the spaces provided in Question-Answer Book B. The Answer Sheet for Section A and the Question-Answer Book for Section B will be collected separately at the end of the examination.
-
A Periodic Table is printed on page 20 of Question-Answer Book B. Atomic numbers and relative atomic masses of elements can be obtained from the Periodic Table.
INSTRUCTIONS FOR SECTION A (MULTIPLE-CHOICE QUESTIONS)
-
Read carefully the instructions on the Answer Sheet. After the announcement of the start of the examination, you should first stick a barcode label and insert the information required in the spaces provided. No extra time will be given for sticking on the barcode label after the 'Time is up' announcement.
-
When told to open this book, you should check that all the questions are there. Look for the words ‘END OF SECTION A’ after the last question.
-
All questions carry equal marks.
-
ANSWER ALL QUESTIONS. You are advised to use an HB pencil to mark all the answers on the Answer Sheet, so that wrong marks can be completely erased with a clean rubber. You must mark the answers clearly; otherwise you will lose marks if the answers cannot be captured.
-
You should mark only ONE answer for each question. If you mark more than one answer, you will receive NO MARKS for that question.
-
No marks will be deducted for wrong answers.
© Hong Kong Examinations and Assessment Authority All Rights Reserved 2021
Not to be taken away before the end of the examination session
Hong Kong Examinations and Assessment Authority
All Rights Reserved 2021
2021-DSE-CHEM 1A-1
Provided by dse.life

真题 Paper 2(英文)
2021-DSE CHEM PAPER 2 HONG KONG EXAMINATIONS AND ASSESSMENT AUTHORITY HONG KONG DIPLOMA OF SECONDARY EDUCATION EXAMINATION 2021 CHEMISTRY PAPER 2 11:45 am – 12:45 pm (1 hour) This paper must be answered in English INSTRUCTIONS (1) This paper consists of THREE sections, Section A, Section B and Section C. Attempt ALL questions in any TWO sections. (2) Write your answers in the DSE(D) Answer Book provided. Start each question (not part of a question) on a new page. (3) A Periodic Table is printed on page 8 of this Question Paper. Atomic numbers and relative atomic masses of elements can be obtained from the Periodic Table. ©香港考試及評核局保留版權 Hong Kong Examinations and Assessment Authority All Rights Reserved 2021 Not to be taken away before the end of the examination session 2021-DSE-CHEM 2-1 Provided by dse.life

